If you work within pharmaceutical industry, chances are you are familiar with IDMP (Identification of Medicinal Products) - a set of ISO standards for unique identification of medicinal products. With IDMP becoming a global regulatory requirement, having IDMP-compliant data assets, systems and business processes is becoming essential for leading pharma companies.
However, diverging implementations of IDMP have led the industry into forming a collaborative industry initiative under the wing of Pistoia Alliance, to define the IDMP Ontology, a common semantically concise and industry-oriented implementation of IDMP ISO standards.
IDMP Ontology is a great opportunity for big-pharma to have a holistic cross-function view on the product data assets, while still achieving regulatory compliance. But, where do you start with adopting IDMP Ontology in your organization? Here are some straightforward steps to get started.
Assessment & Concept
Step 1: Determine the scope and key stakeholders
Identify the scope of your implementation: Determine which business functions of your company are affected by IDMP implementation. This could include regulatory affairs, pharmacovigilance, supply chain management, IT and other departments. In addition, it is important to have key stakeholders onboard right from the start.
Step 2: Prioritize Use Case
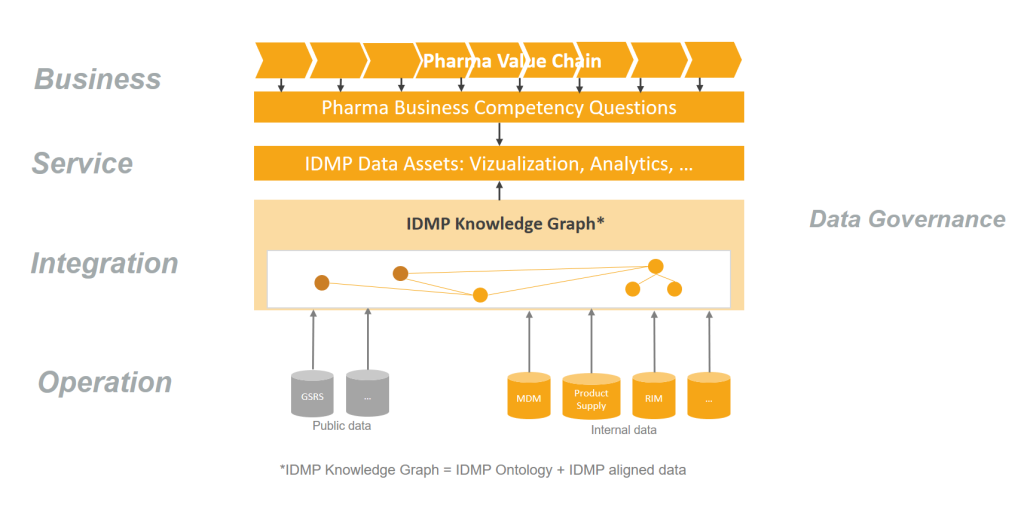
IDMP domains cover the product lifecycle throughout the complete pharma value chain. Prioritized use cases should help keep the implementation focused and make sure demonstrable results are available early on. Ideally, they should be broken down into concrete competency questions such as “In which manufacturing steps is substance <S> used?”.
Step 3: Capability Assessment
Assess your current data assets, IT systems, business processes and organization to identify gaps and areas that need improvement. Evaluate IDMP maturity level at your company and take a holistic view on all existing digitalization programs. Frameworks such as DCAM can be very helpful to conduct capability assessment in a structured and complete way.
Step 4: Implementation Concept and Plan
Out of the Capability Assessment results, outline a TO-BE state that should address found gaps. Depending on the maturity level of your organization, this step may include refinement of technical and business architecture and even vendor selection. Lastly, outline a plan for your IDMP implementation journey including important milestones, responsibilities and budget.
Implementation & Governance
Step 5: Proof of Concept: IDMP Ontology Alignment
Once the relevant data sources are identified for the prioritized competency questions (e.g. RIM, MDM, Substance registry…), internal terminology can be aligned to the IDMP Ontology. By integrating data into IDMP Knowledge Graph, results of IDMP aligned data can quickly be visible.
Step 6: Implement the solution
While PoC implementation (Step 5) is important to relatively quickly demonstrate value and convince stakeholders, a toolset to facilitate IDMP data assets, which is scalable, easy-to-use, cost-effective and compatible with your existing systems still needs to be stablished. Work with your vendor or implementation partner to configure and implement a fit-for-purpose IDMP solution.
Step 7: Establish Data Governance around IDMP
In order to maintain semantically correct and linked data assets, it is crucial to ensure tools and processes for proper metadata, reference and master data management. Establish roles and responsibilities, define data domains ownership, and provide training for your staff on the new systems and processes.
Step 8: Expand IDMP Knowledge Graph
Established IT infrastructure and governance processes for IDMP data assets allow for further expansion and enrichment of the IDMP Knowledge Graph to further data sources and functional business areas. Continuously onboard further use cases, stakeholders and systems, to leverage the value of linked IDMP data beyond mere compliance.
It is important to keep in mind that IDMP implementation can be a long-term project that requires ongoing commitment and investment, as well as alignment with ongoing digitalization initiatives. Therefore, it is important to involve key stakeholders and subject matter experts in the planning and implementation process, but also to work with a trusted and experienced implementation partner to ensure a successful outcome.
Contact our experts and check out how OSTHUS can support your IDMP Ontology adoption journey!





